Suche
Lesesoftware
Specials
Info / Kontakt
Ecology of Freshwaters - Earth's Bloodstream
von: Brian R. Moss
Wiley, 2018
ISBN: 9781119239437 , 560 Seiten
5. Auflage
Format: ePUB
Kopierschutz: DRM




Preis: 68,99 EUR
eBook anfordern 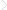
1
The world as it was and the world as it is
1.1 Early ecological history
Our planet is old, around 4.53 billion years on current estimates, but we humans are very young. Only about 100 000 years have passed since we emerged distinctively as Homo sapiens from our previous ancestors. They had had comparatively little effect on the planet, and so did we until the last 15 000 years or so. Before then, the planet changed slowly but continually, under natural geological forces: volcanic eruption, plate separation and continental drift; natural cycles in the Earth’s orbit around the Sun; and small changes in the rate at which the Sun emits energy. Its surface changed just as much because the inevitabilities of evolution were producing a succession of organisms that altered the chemistry of the atmosphere and oceans. Around 2 billion years ago, an atmosphere that had previously been free of molecular oxygen was steadily oxygenated because one group of bacteria, the Cyanobacteria (Fig. 1.1), had evolved the ability to use water as the hydrogen donor needed to reduce carbon dioxide in photosynthesis, and released oxygen as a by‐product.
Figure 1.1 Cyanobacteria, which are now ubiquitous in soils, fresh and salt waters, had a pivotal role in the history of the biosphere. They evolved the ability to use water as a hydrogen donor in photosynthesis, thus releasing molecular oxygen as a by‐product. Individual cells of cyanobacteria (inset) are generally very small (around 1–2 µm) but may aggregate in much bigger filaments and colonies, sometimes occurring so abundantly as to colour the water prominently, as in this temple tank in Nepal. Ancient fossils suggest that the range of forms of cyanobacteria have not changed greatly since they first evolved.
(Reproduced with permission of K. J. Irvine. Inset reproduced with permission of Matthew J. Parker.)
This created problems for a biosphere maintained by anaerobic bacteria, because free oxygen was toxic, but one consequence appears to have been the evolution of the eukaryotic cell, in which, through processes of symbiosis, host cells, probably Archaebacteria, engulfed other bacteria whose enzymes could function deep in the combined cell, away from the increasing oxygen concentrations in the environment. Oxygen then built up steadily in the atmosphere until concentrations were high enough (Fig. 1.2) for diffusion to be able to support bigger, multicellular organisation, between 500 and 600 million years ago. Multicellularity allows specialist systems to develop and was rapidly adopted. Multicellular systems could cope with conditions on land, and a biodiversity previously confined to water was joined by one that could take advantage of very high oxygen concentrations in the air. Oxygen is not very soluble in water (see Sections 5.2 and 5.3). On land there was also a greater supply of light energy (water absorbs the Sun’s radiation very quickly; see Sections 7.2 and 7.3). In turn, these enhanced conditions allowed the eventual evolution of mammals that could start to modify conditions to their own ends through a high brain capacity. We were born.
Figure 1.2 Reconstructed changes in oxygen and carbon dioxide concentrations in the Earth’s atmosphere over geological time. The envelopes indicate the variation calculated from different geological models, but the trends are clear. Major events in evolution are also shown. A bar is the unit of atmospheric pressure. Current total pressure is close to 1 bar (or 100 on the logarithmic scale used).
(Based on Mojzsis 2001.)
The Earth is well supplied with the twenty or so elements that natural selection has used to produce and maintain living systems, but it has limited supplies, in available form at the surface, of some of them. The stock must be recycled. Moreover, liquid water is essential for living cells to function, and, from the end of the Hadean Period 4 billion years ago, when Earth had cooled sufficiently for water to condense from the steamy atmosphere of volcanic gases, there was established a water cycle. The essence of this is that water, evaporated from the oceans and land surfaces through solar heating, moves upwards or polewards in the atmosphere, cools and condenses. It falls as rain or snow, and runs off the land and back to the ocean.
In doing so, it is retained for a time in a continuous system of hilly streams and rivers, groundwaters, pools, wetlands and lakes, and then in floodplain rivers and estuaries that connect them with the coastal seas and oceans (Fig. 1.3). Water dissolves a huge range of substances and carries them with it during the liquid phases of this cycle. Some are absorbed by aquatic organisms; others, like nitrogen compounds, are converted quickly to gases by bacteria and returned to the atmosphere, and yet others contribute to the saltiness of the ocean or are precipitated out into sediments and newly forming rocks. Recycling of these latter elements is long term. Movements of the Earth’s plates against one another raise new mountains over millions of years and weathering slowly re‐releases substances usable by organisms. Recycling of many essential elements, however, has to be much more rapid and depends on biological processes. Water thus acts like a bloodstream for the Earth (Fig. 1.4), its rivers and lakes the equivalents of arteries and veins, its evaporative surfaces a sun‐driven heart that pumps the water around, and its basins, especially lakes and wetlands, its digestive and excretal systems, foci of biological activity that shuttle dissolved substances between organisms and the water.
Figure 1.3 Linkages among parts of the freshwater system, the catchment area of the land, the atmosphere and the water cycle.
Figure 1.4 Seen from space, the freshwater systems of the Earth support the analogy that they are the bloodstream of the biosphere. The many mouths of the River Ganges shown here discharge into the Indian Ocean.
(Reproduced with permission of USGS EROS Data Center Satellite Systems Branch.)
There appears to be some overall linkage of these activities, though we have no idea how it is achieved. The oxygen and carbon dioxide concentrations in the atmosphere and the saltiness of the sea have been maintained for a long time within limits that allow the persistence of liquid water and of multicellular organisms, despite geological forces that could threaten this. Carbon dioxide remained between 190 and 260 ppm by volume for at least a million years until very recently – a period that included a number of advances of the polar and mountain glaciers – and oxygen concentrations have been around 21% for at least as long. Carbon dioxide was prevented from rising much higher through the storage of carbon compounds in waterlogged soils, peats and lake sediments, whilst gases produced by living organisms, such as methane and dimethyl sulphide, react with oxygen and temper its concentration downwards. Much higher oxygen concentrations would promote uncontrollable forest and grassland fires and indeed this had happened earlier in geological history. The chemistry of both seawater and the atmosphere is maintained in a non‐equilibrium state by the activities of living organisms (Table 1.1). Without them, we would have a much hotter planet and possibly no liquid water, and then only with a crushing atmospheric pressure. But how this system is maintained in an apparently orderly way is a mystery. There appears to be cooperation, but cooperation and natural selection do not easily fit together.
Table 1.1 Composition of the Earth’s atmosphere, compared with those of its closest planets, Mars and Venus. Equilibrium Earth is calculated from chemical models that assume that all possible reactions are allowed to run to equilibrium. Present‐day composition is as measured on Earth, and for Mars and Venus is deduced from spectroscopic measurements. (Based on Lovelock 1979. Reproduced with permission of Oxford University Press.)
| Venus | Equilibrium Earth | Mars | Earth as it is |
| Carbon dioxide (%) | 98 | 98 | 95 | 0.03 |
| Nitrogen (%) | 1.9 | 1.9 | 2.7 | 79 |
| Oxygen (%) | Trace | Trace | 0.13 | 21 |
| Argon (%) | 0.1 | 0.1 | 2 | 1 |
| Surface temperature (°C) | 477 | 290 | –53 | 13 |
| Total pressure (bars) | 90 | 60 | 0.0064 | 1 |
1.2 The more recent past
We know a great deal about the ecology of our planet but the detail is greater for the past few million years, and particularly for the last few tens of thousands of years, than for any time previously. This latter time embraces the final melting back of the ice sheets that had advanced and retreated over the previous several million years. As ice advances, it bulldozes the land surfaces, widens pre‐existing river valleys and changes the former courses of rivers. It scrapes out new basins for eventual...







